EFFECT OF OXIDATION IN OUR DAILY LIFE
EFFECT OF OXIDATION IN OUR DAILY LIFE
Q. What is rusting?
Ans - The process of rust formation in presence of oxygen and water or water vapour is called rusting.
🔑Keys points 🗝️
➡️Moisture
➡️Acid: higher pH inhibits the Corrosion of iron.
➡️Salt: Iron tends to rust faster in the sea, due to presence of various salts.
➡️Impurity : Pure iron tends to rust more slowly when compared to iron containing a mixture of metals.
These are affecting the rust.
What is Corrosion?
Ans - Chemical action on the surface of metals by moisture, air or chemicals is called Corrosion.
Ex- black layer on silver, green layer on copper etc.
➡️Rusting of iron is a redox reaction.
What is Rancidity?
Ans :- The process of Oxidation of fats and oils that can be easily noticed by the change in taste and smell is called Rancidity.
Ex- the taste and smell of butter changes when kept for long.
Q. How can be reduced Rancidity?
Ans- Rancidity can be reduced by-
➡️Sotring food in air tight containers.
➡️Storing food in refrigerator.
➡️Adding antioxidants.
➡️Storing food in an environment of nitrogen.
➡️It can be prevented by adding anti - oxidants to foods containing fats and oils.
Q. What is antioxidants?
Ans- It is a chemical substance that slow the rate of oxidation process. It is used to preserve food stuffs and to prevent harmful effects of oxidation in rubber, plastics, paints.
Q. Why do we apply paint on iron articles?
Ans- we apply paint on iron articles to prevent from rusting. When we painted then the contact of iron articles are disconnected from moisture and air. Due to this reason rusting of iron does not occur.
Q. Oil and fat containing food items are flushed with nitrogen. Why?
Ans- Nitrogen is a less reactive element. While oxygen reacts easily with food materials and oxidised and make it rancid. Due to this reason, food items are flushed instead of oxygen and makes it safe.
➡️When copper(shiny brown in Colour ) reacts with oxygen then it form copper oxide which Colour is black.
Q. Why should a magnesium ribbon be cleaned before burning in air?
Ans- Magnesium is a very reactive metal. When it comes to contact with air then it formed magnesium oxide (MgO) on its surface(सतह) this is stable and prevents further(आगे बढाना) reaction of magnesium with oxygen due to this reason magnesium ribbon is cleaned by sandpaper to remove this layer.
>>Types of chemical reactions 👇
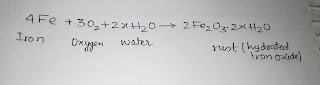

Comments
Post a Comment